Should I Give My Dog Apoquel® To Stop Its Itching And Scratching?
Ron Hines DVM PhD
 What About Apoquel® For My Cat?
What About Apoquel® For My Cat?
Advances in our understanding of the immune system have changed your veterinarian’s medication options for your itching dog. Apoquel®, a Zoetis product, is a breakthrough medication designed to interrupt the inflammatory process that causes itching in dogs with allergies (canine atopy) It does so by blocking the pro-inflammatory cascade. (read here)
In the late 1980s, an Australian chemist isolated two kinase enzyme molecules. At the time, kinases were already known to be important messenger/signaling compounds that the cells within the body use to communicate with one another. There were a very large number of them and scientists didn’t know what many of them did. The Australian chemist jokingly named the two new ones JAKs (for “just another kinase”). But even then, he had an inkling that they might be important. As knowledge of their function increased and two additional ones were discovered, a more serious name for them was substituted – Janus Kinase Inhibitors. They were found to be key players in the control of body growth and development, white and red blood cell formation, immunity, inflammation and policing the body to stamp out tumor formation. The four we currently know of are JAK1, JAK2, JAK3 and TYK2. JAK1 and JAK3 kinases are particularly important for the proper functioning of your pet’s antibody-producing cells (B-cells) and its “policeman” cells (T-cells). (read here)
As soon as the functions of the JAK messengers were understood, pharmaceutical companies went to work developing compounds that might block their actions. They knew that compounds that could block the action of JAKs (Janus Kinase Inhibitors = “jakinhibs”) might be useful in fighting abnormal immune-related processes in people – diseases like rheumatoid arthritis, psoriasis, Crohn’s disease and ulcerative colitis. The company that devoted the most effort was Pfizer. Zoetis is the animal spin-off of the Pfizer Pharmaceutical Company.
JAK inhibitors, like Apoquel® (oclacitinib) for itching in dogs and the one Pfizer markets for humans with RA, ulcerative colitis or psoriasis: Xeljanz (tofacitinib), pharmacists refer to as “small-molecule” pharmaceuticals. That means they are molecules that are small enough to enter the body when you or your pet takes them orally. “Large molecule” drugs like the Humira®, Enbrel® or Remicade® you see advertised on TV must be given by injection because those molecules are too large to be absorbed through your stomach and intestines. JAK inhibitors like Apoquel® target (“neutralize”) several cytokines that are acting as messenger compounds to produce the inflammation occurring in your allergic dog’s skin. With those messenger cytokines blocked, the complex inflammatory process and itching that torments your dog should subside.
I know of six JAK-inhibiting compounds that are under development and examination. Tofacitinib, ruxolitinib, baricitinib, decernotinib, filgotinib, and oclacitinib. These compounds differ in their “head groups”- something that affects their stability and potency within the body and, hopefully, influences which JAKs they target the most.
Sounds Like A Really Great Advancement In Treatment Options For My Dog! Does Apoquel® Work?
Well, it is and, in most cases, it does.
But here is the potential problem you need to be aware of: If you read the link to a scientific paper Zoetis published when it was still part of Pfizer, you will see that Apoquel® does not entirely focus on inhibiting (blocking) interleukin-31 (IL-31), the compound driving your dog’s urge to itch. (read here) or for more detail ask me to send you Gonzales2012) Apoquel® has the potential to reduce the ability of the other JAKs to perform their positive and very important functions. Unfortunately, no small molecule medication yet discovered targets only IL-31. They all have the potential to interrupt the production of many other compounds and processes that defend your dog’s body. That is why the FDA recently changed the label warnings for Xeljanz®, Apoquel’s “sister” human drug.
Veterinarians cannot simply interrupt the production of the four JAKs. They are not something your dog’s body can live very long without. A deficiency of JAK3 leads to increased susceptibility to infections. Adequate JAK2 is necessary to produce red blood cells (erythropoiesis) and a lack of JAK1 leads to neurological (nerve) and lymphocyte abnormalities. Both portions of your dog’s immune system, its antibody system (B cells) and its killer-cell system (Cell-mediated immunity) require that a sufficient amount of JAK3 be present. Theoretically, another large molecule injectable drug Zoetis produces, Cytopoint®, targets only IL-31. As I already mentioned, in contrast to Apoquel® which is a “small molecule drug”, pharmacists call Cytopoint® and drugs like it “large molecule drugs” (=”Biologics”) because the molecules of those drugs are too big to be absorbed intact through you or your dog’s intestine. So, all must be given by injection. Cytopoint®, unlike Apoquel®, is a monoclonal antibody (mAb).
Why Might Some Dog Owners Consider Apoquel®?
No one is really happy with the safety and side effects of oral corticosteroids or Atopica®, the only other effective treatment options for itching available for your dog until now. The side effects of both steroids and Atopica® are substantial and dose-size and length-of-time on the medication dependent. Antihistamines, lotions, desensitization shots & drops, antibiotics supplements and special diets although usually safe, are often minimally effective (if at all) in the treatment of the canine allergies underlying your dog’s chronic itching. see here: They are also very time-consuming and for larger dogs superficial treatments can present physical challenges to their owners.
They are also very time-consuming and for larger dogs superficial treatments can present physical challenges to their owners.
Oral and Injectable Steroids (corticosteroids, prednisone, prednisolone, triamcinolone, etc.) are all quite dramatic in blocking the itch and allowing your pet’s traumatized skin to heal. But their prolonged use at effective doses often causes worrisome side effects: weight gain, excessive water consumption and urination, elevation in liver enzymes, behavioral changes, weakened ligaments, muscle loss, thin skin, blood cell changes, etc. Those meds are also implicated as triggers for pancreatitis, Cushing’s disease and diabetes. Although less dangerous, corticosteroid-containing sprays and lotions are only partially effective on their own when they are applied topically. Topical tacrolimus ointment often helps resolve localized skin lesions. But it’s not generally effective on its own in cases related to generalized skin allergies. (read here) Fatty acid supplements seem helpful for some patients. But they do not address the underlying causes of canine allergic skin disease or itching.
I am also not an enthusiastic fan of skin tests or blood tests to identify possible allergens in your dog’s environment because I do not believe that these tests generally lead to benefits that are sufficient to justify the procedures. Read about that here. It is true that desensitization, if successful, avoids exposing your pet to the side effects of powerful drugs. But I have just not been impressed by the results of those desensitization procedures in the majority of allergic dogs. If you elect to go that rout, you might consider having your veterinarian cut to the chase with a commercial, prepared for your region, allergen mixture such as Respit®. Veterinary dermatologists might respond: “Well, even if your dog must continue on its current anti-itch medications and frequent baths, you will be able to give them less of its medications or “if we had not gone through the desensitization procedure your pet’s problem would be worse by now”.
Food Allergies tend to be over-diagnosed in itchy dogs. Dog food companies encourage that belief and heavily market those products with deceptive claims. Hypoallergenic and novel protein diets are occasionally, but infrequently, helpful in canine atopy cases. Considerably more so when the problem is diarrhea. I would still give them an early try since food allergies do account for, perhaps, 5-8% of dog skin itch problems. Just don’t try to judge their benefits when other forms of therapy are ongoing. Food intolerances in dogs are considerably more common – but considerably more likely to result in digestive disturbances and diarrhea than in itchy skin.
Antibiotics for the secondary staph skin infections that usually accompany canine allergic skin disease do offer many pets temporary relief. But repeated or persistent use of antibiotics is an invitation for the introduction of antibiotic-resistant bacteria into your household. (read here) Regardless of your hygienic attempts to prevent it, resistant mutant bacteria strains that persistent or intermittent antibiotic use generate in your pets. These bacteria often spread to human family members and vice versa. (read here, here, here & here) Nevertheless, pharmaceutical companies still intensively market antibiotics to veterinarians and the owners of itchy pets. See here:  Surface antiseptic products accomplish much the same thing but without that inherent danger.
Surface antiseptic products accomplish much the same thing but without that inherent danger.
What Can You Tell Me About The Drug, Apoquel®?
As I mentioned, Apoquel® (oclacitinib) was developed by Pfizer and is sold to veterinarians today by its veterinary spin-off Zoetis. Apoquel is relatively selective for disabling a signaling or instructing protein JAK1, one of whose functions is to stimulate the production of inflammatory (think itchy) cytokines. Little is known as to the long-term side effects of disabling JAK1. (read here)
Pfizer had invested more into advancing the understanding of the role of JAKs and into engineering new JAK inhibitors than any other company. When Zoetis, looked for opportunities to benefit from Pfizer’s research depth, they saw canine skin allergy treatment as filling a definite need and being quite lucrative as well. You have your client for life. Estimates are that 8.2 million canine pets suffer from allergic skin disease – 10% of the US dog population.
Apoquel® is rapidly absorbed and fast acting (within a day). Its particular structure was engineered in an attempt to have its greatest inhibitory effect on your dog’s JAK1 system because Pfizer research indicated that JAK1 activated many of the processes causing your pet’s discomfort (scratching, rubbing, chewing). Those processes are thought to be due to the liberation of certain other cytokines, particularly interleukin-31. It was hoped that concentrating Apoquel’s activity on JAK1 would allow the other JAKs to continue their important roles such as JAK2’s beneficial effects on blood cell formation. Read in detail about that here. Ask me for the full article. Apoquel® was launched and available to dog owners in 2014. In September of 2014, Zoetis began severely limiting its distribution. The official reason given was “production challenges”. By April, sufficient supply was available again. In succeeding years, there has been no disruptions in supply. Zoetis does not recommend using Apoquel® at the same time that corticosteroids or Atopica®/cyclosporin are being given to your dog. They say they have not accumulated negative data regarding the use of those treatments combined with Apoquel; but that the possible combined effects have “not been investigated in any meaningful way so far”.
I Don’t Want To Read Some Promo – I Want Unbiased Information About Apoquel®
Very little unbiased information is available to you on the internet or elsewhere. I do not know of any sources other than this site that is written by a non-stakeholder. That is today’s new norm in the veterinary pharmaceutical industry and academia. All published studies I know of were funded by Pfizer or Zoetis or conducted by their scientific employees in-house – all with no outside neutral oversight. You can read a key Zoetis-sponsored study here. Scroll down to Competing Interests. When commercial interests rather than simple academic curiosity are your only source of information on anything, it is next to impossible to separate fact from hype. Any data, honestly obtained, can be presented in different ways with the goal being to prove a point. Portions can be deleted that are unfavorable. Tests can be repeated until the desired results are obtained. Experimental designs can be consciously or unconsciously manipulated to one’s advantage and to fit one’s goals. It has been my experience that the best place to get unbiased information about something is not from someone financed by a company that has something it wants to sell to you or your veterinarian. You can view all of the companies that have a dog in this race here.
Elvis once said, “Truth is like the sun. You can shut it out for a time, but it ain’t goin’ away.” I am going to try to speed Elvis’ process along a bit. If you have had good or bad experiences using Apoquel® in your dog, please let me know what they were and I will put your anonymous responses here. Pet owners and veterinarians can report side effects (adverse events) to the FDA or to the product’s manufacturer. They rarely do. It is estimated that only about 1% of human adverse drug events are ever reported to the FDA.
Are All Itching Dogs Good Candidates For Apoquel®?
No.
Dogs under one year of age did not react well to Apoquel®. Zoetis also suggests that Apoquel® not be given to dogs intended to be bred, pregnant or nursing dogs nor to dogs receiving other medications that affect their immune system. Due to Apoquel’s immunosuppressive qualities, it should not be given to dogs with known cancer or mange. I would also suggest that this medication should not be given to itchy pets until less drastic treatments and modifications to your dog’s lifestyle have failed. That includes extremely strict flea control, time-occupying activities for dogs that suffer from boredom, behavior modifying techniques for those that lick from separation anxiety, diet trials, feeding schedule modifications and the like.

What Do The Four JAKs Normally Do?
(most readers will do just fine to skip this part)
As I mentioned earlier, the four JAKs are messenger molecules that deliver instructions and news to cells throughout the body. When those body cells receive messages, they respond in pre-programmed ways. As I also pointed out, there is a great deal that we do not yet know about all the processes that are under their control or the consequence of interrupting their messages.
JAK1
Apoquel® was designed to be most effective at blocking the production of IL-31. IL-31 triggers the release of JAK1 and JAK2 pathways. (read here) Mice that produce no JAK1 die before birth or shortly thereafter. Some theorize that they die because they cannot nurse. We also know that JAK1 is important in programmed cell death (apoptosis) and in the constant surveillance required to destroy abnormal cells and cells that have become cancerous before they form discrete tumors. So certain chemicals and environmental contaminants that are known to be tumor-stimulating (carcinogenic) could, conceivably, be more so in animals with reduced JAK1. (read here) JAK1 is also an important messenger in the IgE processes needed to destroy virus, bacteria, fungi and parasites invading your pet. This is the process that, we think, has gone awry in allergic dogs. (ask me for Haan2008.pdf)
JAK2
JAK2 is a messenger involved in the growth of tissue. It appears to coordinate the growth of individual cells in an organ by transmitting messages between individual cells within the organ. As such, it is important in coordinating the production of bone marrow stem cells that have the potential to become red blood cells, white blood cells or blood platelets. Scientists do not yet know all the processes that JAK2 is involved in. But embryonic mice lacking JAK2 messenger compounds die midway through pregnancy due to a lack of red blood cells. Adult mice with a JAK2 deficiency have problems producing milk.
JAK3
JAK3 seems to confine its regulating activities to the immune cells that produce your dog’s antibodies (B-cells) and those that attack things (T-cells) that the pet’s body – rightly or wrongly – has decided are foreign invaders. You can gain some insight into what a lack of JAK3 might do by looking at dogs (and children) that cannot perform those activities due to breaks elsewhere in this complex process. They are at very high risk of infectious diseases (SCID). SCID naturally occurred in some Basset Hounds, Welsh Corgies and Jack Russell Terriers, as well as children.
TYK2
TYK2 (tyrosine-protein kinase) is also a critical factor in the proper function of the immune system. Its many roles are still poorly understood – even though it was the first JAK to be discovered.
JAK Cross Talk
As if my previous JAK explanations were not confusing enough, the four JAKs can apparently, chat with one another in making decisions. (read here & here) So that factor (and perhaps medications like Apoquel®) that influence one JAK member, can have effects on the actions of the other three.
Might You Just Be A Worry Wart? I Know You Expressed Reservations About Atopica® And Over-vaccination Too
I am not overly concerned with the short-term use of Apoquel® in your dog. But I do tend to be cautious – particularly when tinkering with incompletely understood vital body systems when so little long-term safety information is available. For dogs with a 1-2 month-long seasonal itch, for stopgap relief in a fleabite-sensitive pet that picked up fleas, or a dog that just needs fast short-term relief due to an itch flare-up, Apoquel appears to be a suitable option. But we know too little about the long-term effects of this drug for me to be confident about the safety of its use as a long-term solution to your dog’s itch problem. Zoetis should now have client data from dogs that have been taking Apoquel for 5–6 years. I do not believe that information has been shared outside of Zoetis. Generally, corporations quickly release to the public news that is clear-cut and positive. Only one pertinent article appeared in the intervening 6 years. (read here)
All I have is the Freedom of Information Report issued by the FDA when they approved Apoquel in 2013. You can find it online. A deficiency in that report is that only 24 one-year-old beagles were used in the initial testing. I do not know why but, perhaps, pressure on the industry to minimize the use of research animals had something to do with that. Laboratory-bred beagles, particularly young ones, are known for their robust health and strong immune system – not the kind of dog with a quirky immune system likely to be a candidate for Apoquel®. During the 26-week study, some dogs receiving Apoquel® developed viral skin tumors (papillomas), abscesses between their toes and abnormally enlarged lymph nodes. Five treated dogs had microscopic evidence of pneumonia and others showed evidence that their number of protective lymphocytes and bone marrow cells were reduced. A concurrent study in six-month-old beagles given 3 and 5 times the recommended Apoquel® dose was discontinued after four months because of the development of bacterial pneumonia and generalized mange. Studies of new drugs at this stage generally screen those drugs at 10 times the suggested dose, not 3–5 times. (ask me for ICH-M3R2.pdf) So although Zoetis markets this drug as a JAK1 inhibitor targeting IL-31, it appeared to me that the medication was also suppressing facets of the immune system not involved in itching.
Can We Gain Insights From Our Experiences With Similar Drugs?
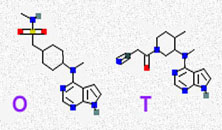
Probably so.
Considerably more data has been accumulated and published regarding another JAK inhibitor similar to Apoquel®: tofacitinib (Xeljanz®). Xeljanz, another Pfizer developed product, began its clinical trials in humans in 2007 as a treatment for rheumatoid arthritis. It is the molecule I marked T in the picture above. O represents the structure of Apoquel. You can see that much of the structure is quite similar. Xeljanz was designed to preferentially inhibit JAK1 and JAK3, and, to a lesser extent, it inhibits JAK2. (read here) More recent studies vary in their conclusions as to its selectivity among the JAK messengers. It can’t be that different from Apoquel because Pfizer mixed it up in an ointment for humans with atopic dermatitis or psoriasis in an attempt to control itching. The FDA granted Pfizer’s request to market tofacitinib in the United States in 2012. The vote was 8 to 2. In 2013, the EMA (the FDA’s European counterpart) denied Pfizer a similar request, stating that the benefits of the drug did not outweigh its risks. In March of 2017, the EMA reversed their decision and approved it.
A problem with interspecies (human to dog or vice versa) comparisons of similar drugs is the shorter life span of dogs and the accelerated tissue damage often associated with shorter life spans. That is a prime reason rats are used to screen medicines for possible long term side effects. The laboratory rat’s average life span is 2.5-3.5 years. So just like hair coat graying, a side effects or drug problems that might take 10–15 years to become apparent in humans might occur much sooner in our dogs. To speed the revelation of those problems, increased dose is an accepted scientific research method. That is why I am concerned with the high-dose side effects and mortality within the beagle study. Sometimes, increased doses over short periods mimics the effects of administration of a lower doses over longer periods. But that is not a hard and fast rule among medications.
Should I Try Apoquel For My Dog Or Not?
We all spend much of our lives making risk/benefit decisions. I currently recommend to my clients that they try Zoetis’ Cytopoint® first. That is because so far, it appears that Cytopoint is more focused on interrupting itch-related IL-31 processes and leaving other systems intact. But when Cytopoint is not an option, I have minimal worries about the short-term use of Apoquel in dogs because the other older treatment options like steroids and cyclosporin/Atopica® are so imperfect. I would be even more inclined to try occasional Apoquel therapy in my dog when other approaches have failed or are impractical.
I would consider Apoquel® for longer periods for my dog, but only when careful monitored. My dog, Maxx is atopic. If Maxx was suffering and unhappy under his current allergy treatment plan, I would consider Apoquel® even if potential side effects turned out to have negative long-term consequences on its health or were life-shortening. That is because I love my dog and would prefer for him to have a somewhat shorter life in happy contentment than a longer one in misery. Effective medications that treat chronic diseases in all of us often present similar dilemmas.
There are inherent dangers in blocking a broadly based, poorly understood, biological process. It might also turn out that certain breeds and certain ages of dogs are better candidates for Apoquel® use than others. I mentioned earlier that certain breeds were found to have idiosyncrasies in their JAK-related processes. Investigators have found immunological differences in other breeds as well. (read here)
I would go with the lowest effective dose of Apoquel® that brought relief to my dog. According to Zoetis: “In a canine flea allergic dermatitis model, oclacitinib (Apoquel®) produced sustained and reproducible anti-pruritic (anti-itch) effects at dosages as low as 0.11 mg/lb (0.25 mg/kg) twice daily.” I would never use Apoquel as a substitute for life changes or prescription medications directed at killing fleas. I would read the owner feedback page on this site from time to time. I would have a baseline blood chemistry panel and CBC run on your dog before beginning Apoquel® so you could compare those baseline results to periods when your dog is consuming Apoquel®. I would consider increased susceptibility to infections, fever, conjunctivitis, diarrhea, warts or weight loss as a red flag warning that the Apoquel® dose or time on the medication needed to be reduced or discontinued. The same goes for liver or kidney test abnormalities. When my dog’s itching had seasonal or intermittent peaks, I would pick the trough (low itch) periods to discontinue Apoquel® in the hope that those breather times from Apoquel® might give my dog’s tumor-scavengering processes a period to work at their full effectiveness. (read here, here & here)
A large number of dogs have been exposed to Canine herpesvirus-1 (CHV-1) during their lifetime (said to be 20% to 98% of dogs, depending on the region). Like all herpes virus, CHV-1 is never completely eliminated from the body – only suppressed. The human JAK inhibitor, tofacitinib/Xeljanz®, is capable of reactivating human herpes virus (Herpes Zoster/shingles). (read here) So I would also be on the lookout for corneal eye problems, vaginal or penile secretions, discharges from the nose or kennel cough-like syndromes in my dog. (read here)
You are on the Vetspace animal health website
Visiting the products that you see displayed on this website help pay the cost of keeping these articles on the Internet.






